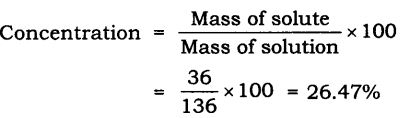On the other hand, heterogeneous mixtures are commonly known as suspensions and colloids.
Mixtures are of two types: homogeneous and heterogeneous. When two or more substances are mixed, but not chemically, a mixture is created. Some commonalities amongst them make them unique. From a solid sphere of mass M and radius R a cube of maximum possible volume is cut. Q 1. Homogeneous Mixtures: The whole mixture is in the same phase. If a single dielectric material is to. Once we get 15 subscriptions with your referral code, we will activate your 1 year subscription absolutely free. Some particles sizes are very small and some are large. Your email address will not be published. Among the following, name a mixture which is used as a food ? Mixtures are divided into two major categories known as homogeneous mixtures and heterogeneous mixtures.
Diffen LLC, n.d. The terms homo and hetero depict the most prominent difference between homogeneous and heterogeneous mixtures.
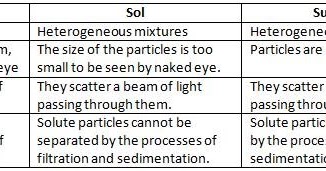
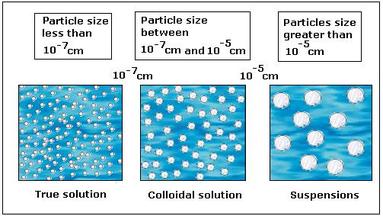
Self Study Material, colloid suspension difference solution colloidal particles solutions between colloids state systems dispersed particle vs solubility mixtures types homogeneous example nm Some examples: mud and water, sand and water. They are bigger than 1 micrometer and are usually large enough to be visible to the naked eye. It cannot be separated easily, Heterogeneous mixture is known as suspensions or colloids. Example: A combination of sodium chloride and sand.
We write on the topics: Food, Technology, Business, Pets, Travel, Finance, and Science". Emulsions:A heterogeneous mixture of two liquids. The size of the particles in the mixtures is very small. Components of homogeneous mixtures cannot be separated easily. Heterogeneous mixtures are composed of the union of two or more substances that do not mix uniformly and can be differentiated with the naked eye. The vision is to cover all differences with great depth. Moment of inertia of cube about an axis passing through its center and perpendicular to one of its faces is : Help me answer: Distance of the centre of mass of a solid uniform cone from its vertex is z0. They could be.
Similarly, if the sample is the entire mixture, you could consider it to be homogenous enough. The homogenous mixture, on the other hand, is solid when the solvent remains solid. (c) What is the other name of homogeneous mixtures? Heterogeneous mixtures are made of two or more substances which show distinctive characteristics. An example is sand in water. . Depending upon the composition of the mixture, it can be divided into two categories. Both the sugar beets and the sand may be seen clearly on their own. As described by the dictionary of Chemistry, a heterogeneous mixture is a combination in which the constitution is not regular and smooth. Difference Between Molecular and Structural Formula, Difference Between Beta Particle and Electron, What is the Difference Between Advice and Guidance, What is the Difference Between Pomade and Paste, What is the Difference Between Outsourcing and Offshoring, What is the Difference Between Facial and Clean Up, What is the Difference Between Judaism and Christianity, What is the Difference Between Resume and CV. Gas in solid: An example of this is hydrogen dissolved in palladium, Liquid in solid: Examples of this include mercury in gold, forming an amalgam, and water (moisture) in salt. Difference Between Homogeneous and Heterogeneous Mixtures, Difference Between Heterogeneous and Homogeneous, Difference Between PayPal Friends And Family And Goods And Services, Difference Between Trading Account and Profit and Loss Account, https://pubs.acs.org/doi/abs/10.1021/ed077p762, https://link.springer.com/chapter/10.1007/978-3-319-15666-8_13, Homogeneous Mixture vs Heterogeneous Mixture, Comparison Table Between Homogeneous and Heterogeneous Mixtures, Main Differences Between Homogeneous and Heterogeneous Mixtures. The most common example of a gaseous solution is the air in our atmosphere, which is nitrogen (the solvent) and has solutes like. Perfume is a liquid aerosol.
Save my name, email, and website in this browser for the next time I comment. A homogeneous mixture has a uniform composition and appearance. What are Homogeneous Mixtures? A mixture is a combination of different substances which retain their own characteristics and can be separated by physical means. Concentration is the amount of solute dissolved in the solvent. It is a mixture that contains components in different phases or has a non-uniform composition. A mixture is said to be homogenous if the substances composition is uniform throughout.
They are those mixtures in which their components are not differentiated at first glance. We may be paid compensation when you click on links to those products and/or services. Salt in water, sugar in water are examples Mixtures of sodium chloride and iron filings and oil and water are examples of heterogeneous mixtures. Even so, each component maintains its characteristics and properties because a chemical reaction does not occur in the union, so they are separable through physical processes. Difference between the Homogeneous and Heterogeneous Mixture. Differentiate between detritivores and decomposers, An Unbiased coin is tossed 4 times. The solid particles do not dissolve in the solvent but are suspended and freely floating. The differences between Homogeneous and Heterogeneous Mixtures are given below: Distribute the referral code to your friends and ask them to register with Tutorix using this referral code. How to download JEE Main admit card without password? The ratio of mass of the solute to the solvent is called the concentration of the solution. Diffen.com. A key feature of suspensions is that the suspended particles settle over time if left undisturbed.
Difference between the Homogeneous and Heterogeneous Mixture A homogeneous mixture is one where its components cannot be differentiated when studied, while in a heterogeneous mixture the components can be differentiated with the naked eye. Example: Sugar and sand mixture, milk, ink, paint, wood, blood, etc. (a) What is a mixture? All the team management, content creation and monetization is handled by Sandeep. Heterogeneous mixtures:It is amixture in which different constituents are not mixed uniformlyand the constituents can be easily seen andcan be easily separated. in the morden periodic table . Difference Between Dell XPS and Dell Inspiron, Difference Between McAfee LiveSafe and Total Protection, Difference Between Honda CR-V EX and EX-L, Difference Between Dell Latitude and Dell Vostro, About Us | Contact Us | Privacy & Cookie Policy | Sitemap | Terms & Conditions | Amazon Affiliate Disclaimer | Careers. In Homogeneous mixtures the composition is uniform throughout the mixture. Since the particles of a heterogeneous mixture are completely visible, they are not uniform throughout. A cereal dish might have doughnuts as a stationary surface and milk as a liquid phase. The main difference between a homogeneous mixture and a heterogeneous mixture is that, unlike heterogeneous mixtures, homogeneous mixtures are consistent, meaning their constitution is the same no matter where one looks. Your subscribed friend will also get 1 month subscription absolutely free. This article may include references and links to products and services from one or more of our advertisers. unit (kg/m3): Sir , how to easily remember the first 20 elements with the atomic no. The features of homogeneous and heterogeneous mixes can be summarised in this way. These types of mixtures can be easily separated.
This uniformity is because the constituents of a homogeneous mixture occur in the same proportion in every part of the mixture. You will understand that the taste is the same irrespective of the sample point. Chromium is added to steel to impart strength and shine. 1. Components of heterogeneous mixture can be separated easily. Expression in S.I. Definition, Composition, Characteristics, Examples, 2. Homogeneous Mixtures:Particle size is often at atomic or molecular level. Rate this post! Molecules are made of atoms that have bonded together. Solutions are homogeneous while suspensions and colloids are heterogeneous.
If the scale of sampling is fine (small), it could be as small as a single molecule. Dissolve sugar in water. Connect with a tutor in less than 60 seconds 24x7. As it turns out, heterogeneous mixtures perform the following functions of homogenization, which is quite interesting. Heterogeneous at the microscopic level but visually homogeneous, Gaseous solutions: When the solvent is a gas, it is only possible to dissolve gaseous solutes in it. P =, My Schooling score was Average and I have scored 68% Percentile in CAT. Homogenization is the method of altering a heterogeneous mixture into a homogenous mixture by using non-soluble fluids such as methanol as a solvent.
But regardless of atomic bonds, mixtures can become quite cohesive. ;What are Mixtures and Solutions?. For instance, water, as well as its aqueous solutions, fall under this category.
As a result of the interaction between the solute as well as the solvent, homogeneous mixtures remain consistentan extremely efficient connection results in very tiny particles of said solute. Examples of heterogeneous mixtures are mud and water, sand, salt and pepper mixture, air etc. The different substances of heterogeneous mixtures are visible to the naked eye. Name the smallest and the largest cell in the human body, Examples of herbs, shrubs, climbers, creepers, What is the molecular mass of glucose molecules (C6H12O6), Find five rational numbers between 2/3 and 4/5. Since the particles of a heterogeneous mixture are completely visible, they are not uniform throughout. It has a uniform composition throughout the mixture. Need explanation for: - Chemical kinetics - NEET, Solve this problem A block rests on a rough inclined plane making an angle of 30o with the horizontal. Colloids are heterogeneous like suspensions but visually appear to be homogeneous because the particles in the mixture are very small1 nanometer to 1 micrometer. Homogeneous and heterogeneous mixtures are the two words one comes across in all of this research. A homogeneous mixture is a solution remaining from the uniform mixture of two or more elements to the point where they cannot be distinguished with the naked eye. So to remain practical, we use this rule of thumb to decide if a mixture is homogeneous: if the property of interest of the mixture is the same regardless of which sample of it is taken for the examination used, the mixture is homogeneous. Even a mixture of oil and water is heterogeneous because the density of water and oil is different, which prevents uniform distribution in the mixture. (b) What is meant by (i) homogeneous mixtures, and (ii) heterogeneous mixtures?Give two examples of homogeneous mixtures and two of heterogeneous mixtures. Not only that, the individual components of the solution can be different states of matter. All of these chemicals are present in the composition in equal amounts. Water with salt, Chlorine dissolved in water, Vinegar dissolved in water, Seawater, Sugar water, etc., are examples of homogeneous mixtures, whereas Soups and broths.